Global Reach
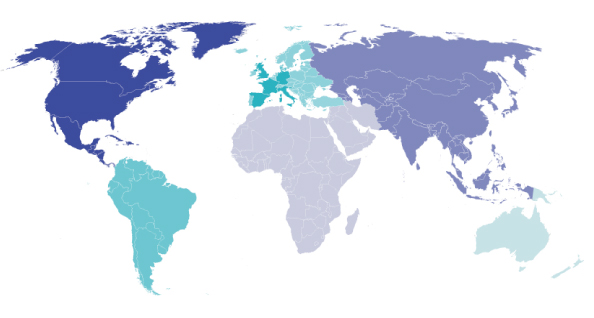
Completed Country Assignments up to 2021
UK 308
Germany 210
France 185
Italy 147
Spain 150
NL 72
Other European 509
Canada 21
USA 29
Latin America 35
Asia 69
Africa / Middle East 18
Australasia 21
UK 308
Germany 210
France 185
Italy 147
Spain 150
NL 72
Other European 509
Canada 21
USA 29
Latin America 35
Asia 69
Africa / Middle East 18
Australasia 21
As of September 2021 we have undertaken, in total, over 1800 country assignments around the world. Most of these (~1300) are in Europe, and of the other 200 country assignments, 23% were in North America, 15% in Latin America (mostly Brazil and Mexico) and 43% in Asia (mostly China and Japan), the rest being in Australia, New Zealand and Africa/Middle East.
PPi has also provided training to payers and health ministries in different countries, such as in 2003 at Amman, Jordan, when Adam Barak led a symposium sharing best practise on healthcare pricing and reimbursement with Health Ministers and officials from over 15 nations across North Africa, Middle East and Asia including Egypt, Saudi Arabia, Pakistan, Lebanon and the UAE.
PPi has always offered its clients a global service, and has undertaken work across the world through our core staff and also through reliable and familiar associates for those countries we are more infrequently asked to assist with. Market access requirements vary enormously across different continents and countries, and so providing our on-the-ground expertise to assist in local matters is a core part of our service offering.
About
TESTIMONIALS
“With their depth of market access experience across the EU, Adam Barak and his team at PPi Healthcare are well-positioned to provide timely, insightful guidance on pricing and reimbursement. ProMetic’s global strategic launch plans involving ultra-orphan therapies, have been greatly enhanced by our collaboration with PPi.”
Kristine Dorward, Director, Marketing & Business Development, ProMetic Life Sciences Inc.
“I want to let you know that the feedback from my colleagues after your presentation was that they are impressed by the amount of information that you have gathered within the research within such a short period.”
Ramona Schmid, Global Market Access, Pricing and Reimbursement Manager, HRA Pharma
“I have worked with PPi on several projects and I will work with them again. The first project was a European pricing study for an orphan product. Having already completed one study with a competitor, I found the PPi team to be particularly insightful, having taken the time to completely understand this complex project.”
Thomas W. MacAllister JD, PhD, Vice President of R&D and General Counsel, Remedy Pharmaceuticals
“Recently I completed an assessment to inform the collection of pharmacoeconomic data in a clinical study in the US and Europe. The team again were very insightful and we had unexpected value-added when we found a member of the PPi had been involved in the pricing and reimbursement activities relating to one of our comparables. Great team, great results!”
Thomas W. MacAllister JD, PhD, Vice President of R&D and General Counsel, Remedy Pharmaceuticals



